C9orf72 Background
In 2011, two groups of researchers from the National Institutes of Health and the Mayo Clinic in Jacksonville simultaneously published findings implicating a mutation in the gene on human chromosome 9 open reading frame 72 (C9orf72) as a cause of many previously unexplainable cases of hereditary amyotrophic lateral sclerosis. The discovery of the C9orf72 mutation, which might explain as many as 40% of familial ALS cases and 9% of sporadic ALS cases, had long eluded researchers because the C9orf72 mutation is different in many ways from other known mutations.
Genes are information encoded in DNA which dictates the production of specific proteins. One reason the C9orf72 mutation was hard to discover is that the mutation is located in an intron of the C9orf72 gene. Genes can be divided into two different types of regions: “exonic” and “intronic”. Introns do not code for actual protein products; exons do. The function of introns is not entirely clear at present, leading some to call introns “junk DNA”. The vast majority of research studies are conducted on exons and consequently, experimental techniques are designed to suit exons. Therefore, because the C9orf72 mutation is located in an intronic region it was far less likely to be discovered.
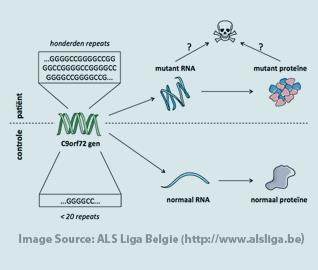
Another reason that the C9orf72 mutation was hard to identify is that most disease-causing gene mutations are single, discrete changes in one “letter” in the DNA sequence of a gene. In contrast, the C9orf72 mutation is a replication of a normally occurring pattern of letters in the DNA sequence of a gene. In the former more typical situation, detection of the mutation is a little like using “spellcheck” to find a misspelled word in a document. In the latter, C9orf72-like situation, detecting the mutation is akin to finding that a properly spelled word is occurring too frequently in a document. Most common sequencing approaches can’t identify and quantify the C9orf72-like mutations. Special techniques were used. Specifically, there is a region in an intron of the C9orf72 gene that in healthy individuals has the DNA nucleotide sequence GGGGCC repeated up to 30 times. In the cases where people harbor mutations, the GGGGCC sequence can be observed as many as hundreds or thousands of times over. This is called the C9orf72 hexanucleotide repeat expansion.
As with every mutation that has been associated with ALS, it remains unclear how the C9orf72 intron hexanucleotide repeat expansion mutation causes ALS. The atypical nature of the mutation begets some atypical observations. Some think that the mutation causes a disruption in the amounts or function of the normal C9orf72 gene product (protein). There is evidence that people harboring the hexanucleotide repeat expansion do, in fact, express less of one specific C9orf72 RNA than normal people. Also, it has been shown that the expanded DNA region results in the production of an expanded RNA. Some scientists have suggested that because of the size and makeup of the expanded C9orf72 RNA, it collapses on itself to form an aberrant three-dimensional structure often referred to as a “G-quadruplex” or “G-quartet”. These large, atypically shaped RNAs may be disruptive to the cell. Still, others have theorized that the expanded C9orf72 RNA leads to the production of repetitive proteins called dipeptide repeats (DPRs) which might also be disruptive to cells. None of the above possibilities is mutually exclusive and other possibilities have not yet been ruled out.
C9orf72 Research Efforts at ALS Therapy Development Institute
The ALS Therapy Development Institute and its scientists are actively focused on the discovery and development of effective treatments for ALS. This includes conducting experiments both clinically and preclinically. Currently, the Institute has a number of staff scientists and technicians conducting experiments in parallel on some of the prominent gene mutations identified to be associated with ALS, including SOD1, TDP43, and C9orf72. Different experiments and approaches are being used to understand the role of these complex genetic mutations in disease onset or progression.
 One ongoing study is designed to inform future therapeutic strategies targeting mutant C9orf72 dipeptide repeat proteins (DPRs). There are many different DPRs created as a result of the C9orf72 mutation in ALS. In order to determine whether a therapeutic has reduced the production of deleterious DPRs or changed their properties, it is essential to first develop ways to detect and quantify the specific DPRs. Scientists at the ALS Therapy Development Institute are conducting a study to characterize all commercially available antibodies designed and reported to detect the possibly pathogenic DPRs. We believe the findings may help inform researchers worldwide on which antibodies to use to identify specific DPRs created by the C9orf72 hexanucleotide expansion in ALS. Institute scientists will present the current results of this study at the 10th Brain Research Conference in Chicago, IL, USA focusing on RNA Metabolism in Neurological Disease.
One ongoing study is designed to inform future therapeutic strategies targeting mutant C9orf72 dipeptide repeat proteins (DPRs). There are many different DPRs created as a result of the C9orf72 mutation in ALS. In order to determine whether a therapeutic has reduced the production of deleterious DPRs or changed their properties, it is essential to first develop ways to detect and quantify the specific DPRs. Scientists at the ALS Therapy Development Institute are conducting a study to characterize all commercially available antibodies designed and reported to detect the possibly pathogenic DPRs. We believe the findings may help inform researchers worldwide on which antibodies to use to identify specific DPRs created by the C9orf72 hexanucleotide expansion in ALS. Institute scientists will present the current results of this study at the 10th Brain Research Conference in Chicago, IL, USA focusing on RNA Metabolism in Neurological Disease.
In parallel to the characterization and validation of antibody reagents for DPR detection, ALS Therapy Development Institute scientists have developed models in human cell lines which can produce C9orf72 DPRs. These will be used as tools to better understand whether DPRs are generally toxic in mammalian cells and how they might exert their toxicity.
In another series of experiments, ALS Therapy Development Institute scientists are optimizing systems designed to detect and quantitate C9orf72 hexanucleotide expansion G-quadruplex RNAs in a mid to high throughput context using small molecule fluorescent probes. As with the DPRs, in order to determine whether a therapeutic has disrupted or reduced the presence of possibly pathogenic G-quadruplex RNAs, it is essential to be able to specifically detect and quantify those G-quadruplex RNAs.
Next, ALS Therapy Development Institute scientists have taken steps to in-license, characterize, and utilize transgenic mouse models of C9orf72-mediated ALS. Institute scientists believe that whole animal models of the disease will likely be essential for an adequate understanding of the disease that will ultimately yield therapeutics.
Finally, Institute scientists are using induced pluripotent stem cell technologies to scale up and differentiate C9orf72 mutant cell lines developed thanks to patient involvement with the Precision Medicine Program.
ALS Therapy Development Institute will continue to study C9orf72 diligently while applying our high scientific standards. We will attempt to objectively put pieces of the puzzle together as fast as we can and apply our findings toward the development of therapeutics for ALS.
Fernando G. Vieira, MD, Beth Levine, and Valerie Tassinari of the Research & Development Team at the ALS Therapy Development Institute contributed substantially to the writing and editing of this article.